GFAP Mouse Monoclonal Antibody
| Cat Number: | MAB-12029 |
|---|---|
| Conjugate: | Unconjugated |
| Size: | 100 ug |
| Clone: | GA5 |
| Concentration: | 1mg/ml |
| Host: | Mouse |
| Isotype: | IgG1 |
| Immunogen: | Purified porcine spinal cord GFAP |
| Reactivity: | Hu, Pig, Ch, Rt, Ms |
| Applications: | Western blot: 1:500 – 1:1000 |
| Molecular Weight: | 50kDa |
| Purification: | Purified |
| Background: | Glial Fibrillary Acidic Protein (GFAP) was discovered by Amico Bignami and coworkers as a major fibrous protein of multiple sclerosis plaques (1). It was subsequently found to be a member of the 10nm or intermediate filament protein family, specifically the intermediate filament protein family Class III, which also includes peripherin, desmin and vimentin. The GFAP protein runs on gels as a ~50kDa protein, usually associated with somewhat lower molecule weight bands which are alternate transcripts from the single gene. The HGNC nomenclature for this protein is, perhaps not surprisingly, GFAP. GFAP is strongly and specifically expressed in astrocytes and certain other astroglia in the central nervous system, in satellite cells in peripheral ganglia, and in non-myelinating Schwann cells in peripheral nerves (2,3). It is also a component of neural stem cells. |
| Form: | Liquid |
| Buffer: | Liquid in PBS 50% glycerol, 5 mM Sodium Azide |
| Storage: | At 4°C short term or -20°C long term. Avoid repeated freezing and thawing |
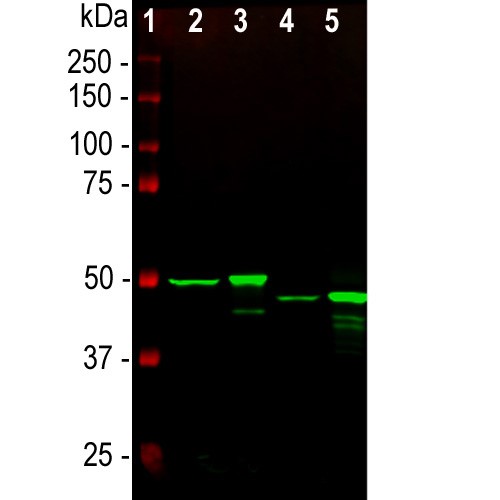
Western blot analysis of whole tissue lysates using mouse mAb to GFAP, dilution 1:1,000, in green: [1] protein standard (red), [2] rat brain, [3] rat spinal cord, [4] mouse brain, [5] mouse spinal cord. The strong band at about 50kDa corresponds to the GFAP protein

Immunofluorescent analysis of rat cerebellum section stained with mouse mAb to GFAP, dilution 1:1,000, in green, costained with rabbit pAb to neurofilament NF-L dilution 1:1,000, in red. Following transcardial perfusion with 4% paraformaldehyde, brain was post fixed for 24 hours, cut to 45μM, and free-floating sections were stained with antibodies. MAB-12029 stains a network of astroglial cells, while the NF-L antibody labels neuronal cells and their processes.

GFAP antibody [GA5] – Astrocyte Marker MAB-12029 immunocytochemical detection in stimulated Cor1 cells. Stimulated Cor1 cells were fixed in formaldehyde, permeabilized, blocked in 1% BSA for 10 mins @ rt°C. Primary Antibody MAB-12029 incubated at 1/1500 for 2 hours in TBS/BSA/azide/0.3% triton. Secondary Antibody: anti mouse IgG Conjugated to: Alexa Fluor® 488 (1/1000).
PROCEDURE OF IMMUNOFLUORESCENT STAINING OF FREE-FLOATING BRAIN TISSUE SECTIONSTISSUE PREPARATION:
1. Perfuse transcardially the animal (rat or mouse) with ice-cold PBS (pH7.4), followed by freshly made 4% paraformaldehyde fixative solution in PBS.
2. Postfix the removed brain in the same 4% paraformaldehyde fixative solution in PBS (4°C for 16 – 24 hours).
3. Cryoprotect the tissue by immersing it in sucrose solutions in PBS (15%, for 24 hours followed by 30% until tissue will sink, may take from 48 hours up to 1 week).
4. Cut 40 – 50μm sections on a cryostat.
5. Keep sections in PBS + 0.05M NaN3 at 4°C
until they were taken for staining. During the staining process, the sections should never be allowed to dry out.
IMMUNOFLUORESCENT STAINING:
1. Rinse sections with PBS
2. Block and Permeabilize sections in 10% Normal Goat Serum (serum of the species the secondary antibody was made in), 1% Triton-100 in PBS for 1 hour with slight agitation.
3. Incubate sections with the primary antibody diluted in 1% Normal Goat Serum , 0.1% Triton-100 in PBS at 4°C overnight with slight agitation.
4. Rinse sections 3 times with PBS, first rinse is quick, but wait 5 minutes between each subsequent rinses. (This step is to wash away unbound primary antibody).
5. Add fluorochrome-conjugated secondary antibody, diluted 1:2,000 in 1% Normal Goat Serum , 0.1% Triton-100 in PBS, and can add Hoechst 1:2,000 (blue dye, for nuclear DNA staining). Cover plate with foil and incubate 2h at room T with slight agitation.
Note: a common example would be ALEXA Fluor anti-mouse, anti-rabbit, or anti-chicken antibodies.
6. Rinse sections 4 times with PBS, first rinse is quick, but wait 5-10 minutes between each subsequent rinses.
7. Mount sections on clean glass slides with glycerol-base mounting medium, and cover it with coverslip. Store slides at 4°C.
References
1. Debus, E. et al. (1983). Differentiation 25(2): 193-203.
2. Achtstätter, Th. et al. (1986) Differentiation 31 : 206 227.
3. Trivino, A. et al. (1992). Vision Res. 32(9): 1601-1607.
4. Mena, MA. et al (1999). J Neural Transmission 106: 1105-1123.
5. Mennini T., Bigini P., Cagnotto A., Carvelli L., Di Nunno P., Fumagalli E., Tortarolo M., Buurman W.A., Ghezzi P., Bendotti C., (2003) Glial activation and TNFR-I overexpression precedes motor dysfunction in the spinal cord of mind mice, Cytokine “in press”.
6. Fumagalli E., Bigini P., Barbera S., De Paola M., Mennini T., 2005 Laboratory of Receptor Pharmacology Mario Negri Institute for Farmacological Research, available on line on www.sciencedirect.com.
